- Individual can clinically manifest only when homozygous (have two copies of the mutant allele).
- In heterozygous situation (one copy of the mutant allele is present) an individual is a carrier of mutation.
- Affects male and female gender equally.
- If both parents are carriers of the mutant allele, the probability is 25% chance of affected children (homozygous), 50% of children have chance of carriers and 25% chance of children having no mutant allele.
Examples:
- Sickle cell disease
- Cartilage hypoplasia
- Congenital insensitivity to pain
- Diastrophic dwarfism
- Gaucher disease
- Hurler syndrome
- Hypophosphatasia
- Manteaux syndrome
- Alkaptonuria
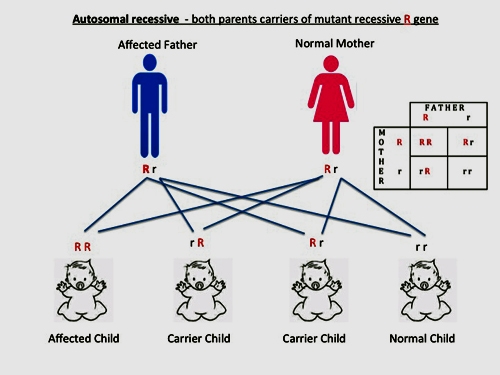
Figure 4. Schematic diagram and Punnett Square showing inheritance pattern in autosomal recessive genetic disorder.