Paget’s disease
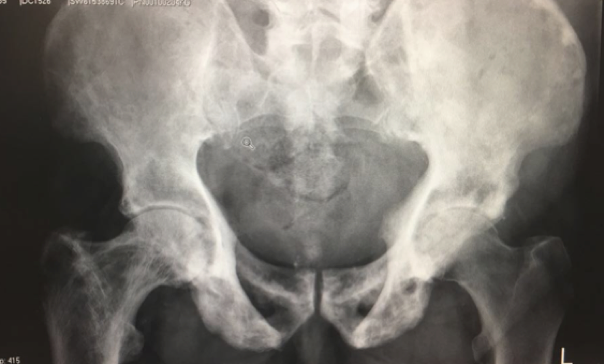
Question: Describe the above radiograph. What is Paget’s disease and its underlying pathophysiology
Answer:Paget’s disease was first described by Sir James Paget in 1876. Aetiology of Paget’s disease remains unknown although this has been associated with positive family history and virus (paramyxovirus, RSV). Primary abnormality is increase in osteoclastic reabsorption of bone followed by compensatory bone formation (accelerated bone remodeling). Bone is rapidly laid down and resorbed. This leads to newly formed bone that is coarse fibred and disorganised architecturally. This leads to mechanically weak bone and the marrow tends to become fibrouswith scanty haematopoietic cells. The abnormal bone is rapidly resorbed by osteoclasts and bone turnover is greatly increased. There are two forms of Paget’s disease, the monostotic and polyostotic form.
Question: How does a patient with Paget’s disease normally present? What are the investigations of choice
Answer:Patient is normally asymptomatic from Paget’s disease. Most patients present with incidental findings on X-rays or raised blood alkaline phosphatase level for investigations of other conditions. Bone pain can be main presenting complaint, related to acute fracture or degenerative joint diseases. X-rays should be requested for acute bony pain in Paget’s disease to rule out acute fracture. This should be supplemented with other radiological investigations such as CT, MRI or bone scan as appropriate. Patient with long-standing Paget’s disease and presenting with acute bony pain should be investigated for acute sarcomatous changes.
Question: If patient with Paget’s disease presents with painful hip/knee, how would you approach and manage the patient?
Answer:History taking and examination form my initial evaluation of the patient. X-ray of the joint or bone involved to rule out fracture or degenerative joint disease. Assuming that conservative management has been exhausted, I would offer the patient total hip replacement (THR)/total knee replacement (TKR) provided patient understands the risks and benefits involved with the procedures. If patient has a deformed limb or bone on X-rays, then long leg alignment view will be helpful in preoperative planning such as osteotomy level to enable the bone to accommodate the prosthesis/implants.
Question: What are the challenges of surgical management of hip/knee arthritis in patient with Paget’s disease?
Answer:The challenges can be divided into local and systemic factors:
- Local factors: deformed bone, bleeding due to increase blood flow, sclerotic bone causing suboptimal position of prosthesis, fracture, protrusion acetabuli, heterotopic ossification, placement of extramedullary jig in TKR due to tibia deformity. A course of bisphosphonate therapy preoperatively is advocated by some to reduce the risk of bleeding.
- Systemic: increased cardiac output, spinal stenosis (anaesthetic challenges).
Further reading
Merkow RL,Pellicci PM,Hely DP,Salvati EA.Total hip replacement for Paget's disease of the hip. J Bone Joint Surg Am 1984; 66(5): 752–758.
Lewallen DG.Hip arthroplasty in patients with Paget's disease. Clin Orthop Relat Res 1999; (369): 243–250.
Question:What are the challenges in management of acute fracture in a patient with Paget’s disease?
Answer: The challenges can be divided into local and systemic factors.
- Local factors: deformed bone, bleeding due to increase blood flow, sclerotic bone causing suboptimal position of prosthesis, fracture.
- Systemic: increase cardiac output, spinal stenosis (anaesthetic challenges).
Further reading
Parvizi J,Klein GR,Sim FH.Surgical management of Paget's disease of bone. J Bone Miner Res 2006; 21 (Suppl 2): P75–P82.
Question :What kind of THR would you offer for patient with Paget’s disease?
Answer: A systematic review by Hanna et al. showed uncemented total hip replacement has lower failure rate in those with Paget’s disease. Postoperative functional outcome is largely similar to patients without Paget’s disease although revision rate higher with aseptic loosening. However, the study concluded that no modern stem design has been fixed using current generation of cementing techniques.
Osteopetrosis
Question: What is osteopetrosis and its underlying pathophysiology?
Answer: Osteopetrosis is a rare congenital disease caused by defective osteoclastic resorption of immature bone. This condition affects one in three million of the population. The inability of osteoclast to acidify Howship’s lacuna (osteoclast dysfunction with lack of ruffled border) leads to dense bone and obliterated medullary canals. This predisposes to fracture with lower extremity affected more than upper extremity and axial skeleton.
Question: What are the orthopaedic manifestations of osteopetrosis?
Answer:
- Head: cranial nerve palsies (skull foramina overgrowth), osteomyelitis (lack of white blood cells)
- Spine: lower back pain due spondylolysis
- Pelvis: coxa vara
- Extremities: carpal tunnel syndrome, long bone fractures
Question: What are the challenges in THR/TKR for patient suffering from osteopetrosis?
Answer:
- Long bone deformities that may require osteotomy prior to insertion of implants
- Dense bone: hard to ream and broach due to narrow/no medullary canal, suboptimal placement of prosthesis leading to higher failure rate, fracture
Further reading
Strickland JP, Berry DJ. Total joint arthroplasty in patients with osteopetrosis: a report of 5 cases and review of the literature.Arthroplasty 2005; 20(6): 815–820.
Landa J, Margolis N, Di Cesare P. Orthopaedic management of the patient with osteopetrosis.J Am Acad Orthop Surg 2007; 15(11): 654–662.
Question: What are the challenges for fracture fixation in a patient with osteopetrosis?
Answer:Breakage of drill bits, bone necrosis due to heat produced during drilling of bone, risk of delay in consolidation and non-union owning to impaired bone remodelling.