- Traumatic injuries to peripheral nerves are defined by the individual structures that are damaged, including the neuron, Schwann cell or the myelin sheath.
Mechanical injury
- Acute compression injuries that do not involve disruption of any of the neural elements.
- It is believed that both mechanical compression of the neural elements and ischaemia are responsible for the resulting loss of nerve function.
- Causes a focal demyelination that results in a focal conduction block. Large myelinated fibres appear more susceptible to ischaemia than smaller fibres.
- When injury is severe enough or prolonged, axonal damage will occur and recovery will be delayed.
Stretch injury
- Stretch-related injuries are the most common. Nerves have some structural elasticity due to the collagen content in the endoneurium but when forces exceed the nerve’s ability to stretch, injury occurs.
- Commonly, continuity is maintained but in severe trauma, the nerve may either be avulsed from its root or be completely disrupted.
- These may be associated with fractures or tractional injuries (for instance in a birth injury, obstetric brachial plexus palsy).
Crush injury
- Focal compressioninjury to the nerve caused by haematomas and/or compartment syndrome following a fracture.
- Neuromal ischaemia leads to subsequent segmental demylination, periaxonal and intramyelin oedema.
Laceration
- Caused by blunt or penetrating injury.
- Nerves are not cleanly sectioned but are damaged in an irregular pattern over a large distance.
- Nerve ends must be cut back to undamaged fascicles for successful repair.
Transection
- Associated with trauma, sharp edge.
- Direct surgical repair of nerve ends with minimal resection of nerve ends.
High velocity trauma
- Damage secondary to rapid tissue expansion in the tract of a missile wound.
- Nerve injury is a result of a large amount of absorbed energy in the surrounding tissue. Example: gunshot.
Cold injury
- Several hours’ exposure to temperatures between –2.5°C and 10°C will slow or stop axoplasmic transport.
- Prolonged exposure may cause damage to peripheral nerves.
Healing injury
- Adherence of nerves to scar tissue, fracture callus may limit nerve gliding.
- The three commonest mechanisms of nerve injury are: (1) stretching injuries; (2) laceration; (3) compression.
Nerve injury classification systems
- Acute peripheral nerve injuries were initially described by Seddon and later refined by Sunderland.
Seddon divided nerve injuries into three categories:
- Neurapraxia is the mildest, does not involve loss of nerve continuity. It is the result of a temporary block to conduction and causes a transient functional loss. Excellent prospects for recovery. No Wallerian degeneration. Focal nerve block. Histopathology shows focal demyelination of the axon shealth without neuromal injury so that distal neural degeneration does not occur.
- Axonotmesis involves a complete interruption of the nerve axon and surrounding myelin but the surrounding mesenchymal structures including the perineurium and epineurium remain intact. Axon and myelin sheath degeneration (Wallerian degeneration) occurs distal to the point of injury, causing complete denervation. Good prospects for recovery as the connective tissue elements provide a framework for healing. Sprouting axons grow down the intact mesenchymal tube to re-innervate the target organ.
- Neurotmesis involves complete severance of a nerve. Functional loss is complete and recovery without surgical repair does not occur because of scar formation and the loss of the mesenchymal guide that directs axonal regrowth and healing.
Sunderland further subdivides axonotmetic injuries into three categories, depending on the severity of injury to the connective tissue:
- First degree – equivalent to neurapraxia.
- Second degree – equivalent to axonotmesis.
- Third degree – effectively axonotmesis combined with partial injury to the endoneurium (scarring). Most variable degree of ultimate recovery.
- Fourth degree – nerve in continuity but only the epineurium is intact. There is complete scarring across the nerve.
- Fifth degree – complete disruption of nerve. Same as neurotmesis.
- First degree injuries have an excellent chance of recovery, second and third degree injuries retain the possibility of functional recovery, depending on the extent of the endoneurial damage. Fourth and fifth degree injuries have no chance of functional recovery without surgical intervention and limited success with repair.
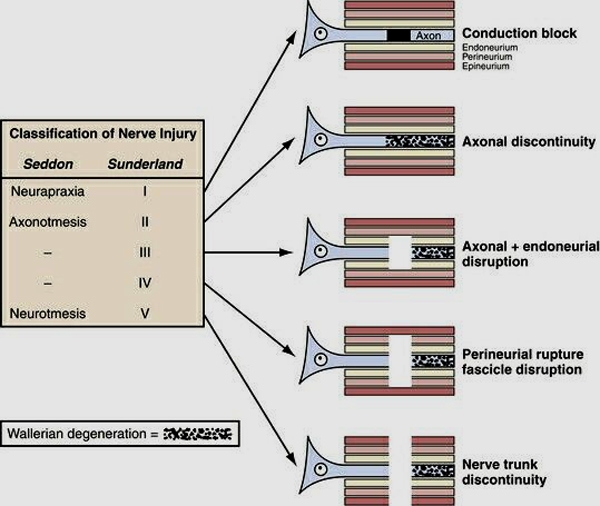
Figure 1. Diagrammatic representation of the five degrees of nerve injury. (1) Segmental demyelination causing conduction block but no damage to the axon and no Wallerian degeneration, (2) damage to the axon severe enough to cause Wallerian degeneration and denervation of the target organ, but with an intact endoneurium and good prospects for axon regeneration, (3) disruption of the axon and its endoneurial sheath inside an intact perineurium, loss of integrity of the endoneurial tubes will limit axon regeneration, (4) disruption of the fasciculi, with nerve trunk continuity maintained only by epineurial tissue, severe limitation of axon regeneration, formation of a mass of misdirected axons (neuroma in continuity), (5) transaction of the entire nerve trunk.